Gene therapy products have been studied to treat various diseases like cancer, genetic diseases, and infectious diseases.
There is a variety of gene therapy products including:
- Plasmid DNA: Circular DNA molecules can be genetically arranged in order to carry therapeutic genes into human cells.
- Viral vectors:Viruses have a natural ability to deliver genetic material into cells, and therefore some gene therapy products are derived from viruses. Once viruses have been altered to remove their ability to cause infectious disease, these modified viruses can be used as vectors (vehicles) to carry therapeutic genes into human cells.
- Bacterial vectors: Bacteria can be modified to prevent them from causing infectious disease and then used as vectors (vehicles) to transmit therapeutic genes into human tissues.
- Human gene editing technology:The goals of gene editing consist of disrupting harmful genes or repairing mutated genes.
- Patient-derived cellular gene therapy products: Cells are removed from the patient, genetically changed (often using a viral vector) and then returned to the patient.
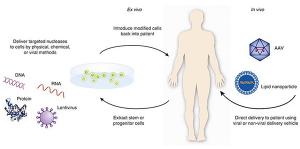
gene therapy products
Moreover, gene therapy products as biological products are administered by the FDA’s Center for Biologics Evaluation and Research (CBER). Clinical studies in humans need the submission of an investigation of new drug application (IND) before initiation of clinical studies in the United States. Marketing a gene therapy product requires submission and approval of a biologic license application (BLA).
Gene therapy product has yet to be verified by the US Food and Drug Administration (FDA), but the recent approval of a rare disease treatment based on an adeno-associated virus (AAV) vector in the European Union sets the stage for similar programs to be presented to the FDA in the coming years. Important new FDA guidance and US law place greater emphasis on therapeutic development and facilitating approvals in rare and orphan disease populations. In lieu of the conventional drug development approach involving early phase safety studies for each indication, several new principles in the recent legislation provide an opportunity to streamline regulatory approval by allowing cumulative existing safety data from related indications to inform the design of a single pivotal clinical study for evaluation of a new therapy.
It is noteworthy to high light approval of the first-gene therapy product Glybera, an AAV vector for treatment of lipoprotein lipase deficiency, by the European Medicines Agency as a salient first step in gene-based drug development. Recent findings in clinical gene therapy trials have now emerged in a variety of monogenic diseases like primary immune deficiencies, hemoglobinopathies, hemophilia B, neurological diseases, ocular diseases, and cancer immunotherapies (excluding oncolytic cancer therapy, which is reviewed elsewhere).
Historic development of gene therapy products in the USA
US Food and Drug Administration (FDA) confirmed the first gene therapy for the US market on August 30th 2017 the. Kymriah, from the pharmaceutical company Novartis, has been developed for treatment of paediatric patients and young people with treatment-resistant acute lymphatic leukaemia. FDA approval proves the administration’s confidence in the safety and usefulness of a gene therapy product, and several other gene therapies may be expected to be approved in the USA and Europe in the coming years.
http://combigene.com/en/market/market-for-gene-therapy/
